Abstract
Differentiation arrest is a hallmark in isocitrate dehydrogenase mutant (mIDH) acute myeloid leukaemia (AML). MIDH enzyme produces d-2-hydroxyglutarate, which inhibits α-ketoglutarate-dependent dioxygenases, including TET2 and Jumonji histone demethylases (J-KDM). The underlying leukemogenic mechanism is unclear and may be due to impaired DNA and/ or histone demethylation. Bivalent chromatin, with both K4me3 and K27me3 marks, maintain genes in a 'poised’ state and is important in modulating expression during cell differentiation. We hypothesised that this may be an important mechanism by which mIDH drives differentiation arrest. Using an mIDH2 inhibitor Enasidenib (ENA), we stimulated granulocytic differentiation of IDH2m AML progenitors (L-Prog), and using a multiomic approach, we tracked epigenetic events that reprogramme arrested L-Prog.
We previously reported downregulation of stem-progenitor signatures and co-incident expression of cell cycle and granulocytic transcriptional networks during ENA-induced differentiation. We also observed increased chromatin accessibility in ENA-treated cells and hypothesised that regulation of H3K27me3 is critical in creating a more permissive epigenetic landscape (Silveira et al., Blood 2021; 138 (Supplement 1): 3467). To test this hypothesis, we performed enhanced reduced representation methylation sequencing and chromatin immunoprecipitation (ChIPseq) assay in parallel with RNAseq. Compared with controls, ENA stimulated global CpG demethylation (mean LogFC=2.01, range 1.79-2.36). However, promoter CpG islands were only modestly hypomethylated in genes upregulated by ENA. This suggests that mechanisms other than DNA hypermethylation are crucial for IDHm leukemogenesis.
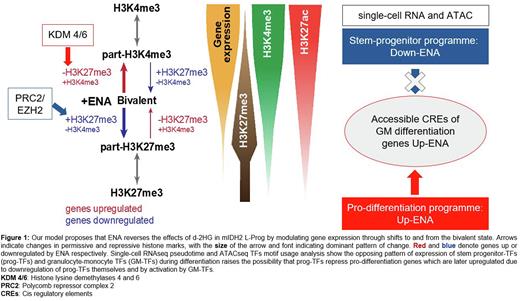
Focusing on histone regulation, we studied H3-K4me3, K27me3 and K27ac marks in ENA-treated L-prog from responsive (R) and non-responsive (NR) samples. We annotated gene promoters as monovalent (mono), bivalent (biv) and partial bivalent (part-K27me3 or part-K4me3), depending on relative enrichment of K27me3 and K4me3 marks, and show correlation between gene expression and repressive (mono-K27me3) to active (mono-K4me3) histone states in a continuum. Consistent with the role of bivalent states in modulating gene expression, the most dynamically differentially expressed genes (DEG, LogFC > 0.25) had the greatest proportion of bivalent promoters. Furthermore, ENA-treated R samples have significantly increased (OR range: 1.11 - 2.17, p < 10e-5) bivalent and part-K4me3 marked promoters respectively compared with DMSO, or with ENA-treated NR samples. 67-84% of ENA-upregulated genes where we detected altered chromatin states transitioned from bivalent to more active partial or monovalent K4me3 states. These transitions were 3x more likely to arise from loss of K27me3 than gain K4me3 (p < 10e-16), suggesting specific enhancement of KDM4/6 activity. Conversely, >95% of downregulated genes moved from relatively activated states (mono-K4me3 or part-K4me3) towards bivalency, mainly through gain of K27me3. These genes were highly enriched with polycomb repressor complex 2 (PRC2)/ EZH2 targets (OR range: 4.28-11.03, p < 0.01).
The switch from part-H3K27me3 to part-H3K4me3 via bivalency in ENA-upregulated differentiation genes is associated with increased H3K27ac, which in turn is correlated with increased chromatin accessibility measured by single cell (sc)ATACseq. Motif usage analysis across the ENA-induced differentiation trajectory revealed that differentiation genes are dual regulated: repressed by stem-progenitor transcription factors (prog-TFs), e.g. GATA2, FOSL1 and MYC which are active in early differentiation, and then activated by granulocyte-monocyte TFs EGR1, CEBPE, JUNB/FOSB and SPI1 in late differentiation, as prog-TFs themselves are downregulated.
Our model (Figure 1) of d-2HG-induced differentiation arrest places modulation of promoter chromatin accessibility and gene expression via KDM4/5 and PRC2-mediated changes to H3K27 methylation centre-stage. For the first time, we show how these chromatin changes drive dynamic transcriptional programmes that initiate and sustain restoration of granulocytic differentiation in primary AML progenitors, and in so doing, identified a novel mechanism of leukemogenesis which may be targetable in other AML subtypes.
Disclosures
Gandhi:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Hasan:Bristol Myers Squibb: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Vyas:Abbvie: Honoraria; Celgene: Honoraria, Research Funding; Astellas: Honoraria; Bristol Myers Squibb: Research Funding; Pfizer: Honoraria; JAZZ: Honoraria; Daiichi Sankyo: Honoraria. Milne:Dark Blue Therapeutics: Consultancy, Current equity holder in private company. Quek:Servier: Research Funding; Bristol Myers Squibb: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal